Abstract
Background: In a previously reported phase 3 head-to-head trial of the Bruton tyrosine kinase (BTK) inhibitors acalabrutinib (Acala) and ibrutinib (Ibr) (NCT02477696), Acala demonstrated noninferiority to Ibr and an improved safety profile with statistically fewer atrial fibrillation/flutter (afib/flutter) events (9% vs 16%) and numerically fewer discontinuations due to adverse events (AEs; 15% vs 21%) vs Ibr in patients (pts) with previously treated CLL (Byrd et al. J Clin Oncol 2021;39:3441-52). To further characterize differences in the safety profile between Acala and Ibr, we conducted a post hoc analysis of AEs using a recently developed, novel statistical method (Ruppert et al. Leukemia 2021;35:2854-61), which considers measures not captured by incidence alone, such as event duration, recurrence, and grade weighting.
Methods: Pts with previously treated CLL and del(17p) or del(11q) received oral Acala 100 mg twice daily or Ibr 420 mg daily until disease progression or unacceptable toxicity. Any-grade AE incidence was reported overall and for AEs commonly associated with BTK inhibitors. An AE burden score was adapted from Ruppert et al and calculated as the sum of the products of the AE duration and AE severity grade (weight) recorded for each all-cause AE, divided by duration of the evaluable treatment-emergent period for each pt. Grade 1-4 AEs were weighted according to their CTCAE severity grades; grade 5 AEs were weighted as 10 to reflect the fatal outcome. Pts with no AE had a score of 0. Separate scores were calculated for grade 1-4 AEs and grade 1-5 AEs.
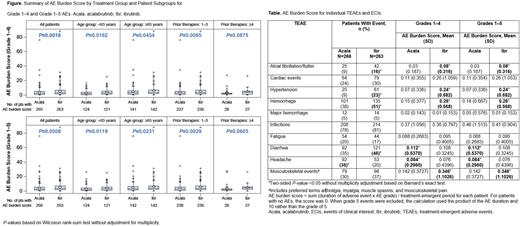
Results: In total, 529 pts (Acala, n=266; Ibr, n=263) received study treatment; median number of prior therapies was 2 in both arms. Median total treatment exposures were 38.3 and 35.5 mo, respectively, at the September 15, 2020 data cutoff. While the frequency of experiencing any AE was similar between Acala (98%) and Ibr (97%), the overall AE burden score considering all AEs was higher for Ibr vs Acala, with (P=0.0006) or without (P=0.0016) grade 5 events included (Figure). The overall AE burden score was higher with Ibr vs Acala in certain pt subgroups, including age <65 y, age ≥65 y, and 1-3 prior therapies; there was no statistical difference among pts with ≥4 prior therapies (Figure). AE incidences and AE burden scores for events of clinical interest (ECIs) and selected AEs are shown in the Table. Among ECIs, the afib/flutter AE burden score P=0.0201and event incidence (P) were higher with Ibr than Acala. The AE burden score for cardiac events overall was numerically higher for Ibr vs Acala, even when including grade 5 events; the incidence rate was also numerically higher for Ibr. For hypertension and hemorrhage, AE burden scores (P<0.0001 and P=0.0007, respectively) and event incidences (P<0.0001 and P=0.0020, respectively) were higher with Ibr; no difference was observed for major hemorrhage. Neither the incidence nor AE burden score for infections was different between treatment arms. Among notable symptomatic AEs, the incidence and AE burden score of fatigue was similar between Acala and Ibr. Diarrhea was more frequent among Ibr-treated pts (P=0.0075); however, the AE burden score was higher with Acala (P=0.0114). Headache was more frequent with Acala (P=0.0002), with a higher AE burden score vs Ibr (P=0.0002). The AE burden score for musculoskeletal events was higher with Ibr (P=0.0229).
Conclusions: The results of this novel analysis using methodology that incorporates event duration and severity weighting further support the superior tolerability of Acala compared with Ibr and confirm the safety differences that have been reported previously between the two drugs. The AE burden score was lower with Acala vs Ibr overall as well as for afib/flutter, hypertension, hemorrhage, and musculoskeletal events, while it was greater with Acala for diarrhea and headache.
Disclosures
Seymour:F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; TG Therapeutics: Consultancy; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genor Biopharma: Membership on an entity's Board of Directors or advisory committees. Byrd:Xencor, Inc: Research Funding; Pharmacyclics LLC: Honoraria, Research Funding; Syndax: Consultancy; Novartis: Consultancy, Honoraria; Kura Oncology, Inc: Consultancy; Janssen Pharmaceuticals, Inc.: Consultancy; Vincerx Pharma: Current equity holder in publicly-traded company; TG Therapeutics: Honoraria; AstraZeneca: Consultancy. Munir:Janssen, AstraZeneca, Alexion, Sobi, Novartis, Roche, Abbvie, Gilead: Honoraria; Janssen, AstraZeneca, Alexion, Abbvie, Novartis, Roche: Membership on an entity's Board of Directors or advisory committees. Ghia:BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; Lilly/Loxo: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Roche: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding. Kater:Janssen, LAVA: Patents & Royalties: Pending; Astra Zeneca, BMS, Roche/Gennetech, Janssen, Abbvie, LAVA: Membership on an entity's Board of Directors or advisory committees; Abbvie, Astra Zeneca, Janssen: Other: Speakers fee; Abbvie, Astra Zeneca, BMS, Janssen, Roche/Genentech: Research Funding; Amsterdam UMC, University of Amsterdam: Current Employment. Furman:AbbVie, AstraZeneca, Beigene, BMS, Genentech, Janssen, Loxo, MEI Pharma, Pharmacyclics, Sanofi, TG Therapeutics, X4 Pharmaceuticals: Consultancy. O'Brien:AbbVie, Alexion, Amgen, Aptose Biosciences, Astellas, AstraZeneca, Autolus, Bristol Myers Squibb, Celgene, DynaMed, Eli Lilly and Company, Gilead, GlaxoSmithKline, Janssen Oncology, Johnson and Johnson, Juno Therapeutics, MEI Pharma Inc, Merck, NOVA Resea: Consultancy; Acerta, Alliance, Beigene Ltd, Caribou Biosciences Inc, Gilead, Kite, Loxo Oncology, Mustang, Nurix Therapeutics Inc, Pfizer, Pharmacyclics, Regeneron, Sunesis, and TG Therapeutics.: Research Funding. Brown:Acerta/ Astra-Zeneca: Consultancy; Abbvie: Consultancy; Pharmacyclics: Consultancy; SecuraBio: Research Funding; Gilead: Research Funding; Sun: Research Funding; TG Therapeutics: Research Funding; Grifols: Consultancy; Pfizer: Consultancy; Beigene: Consultancy, Research Funding; MEI Pharma: Consultancy, Research Funding; Genentech/Roche: Consultancy; Eli Lilly: Consultancy, Research Funding; Catapult: Consultancy; Hutchmed: Consultancy; Bristol-Myers-Squibb/Juno/Celgene: Consultancy; iOnctura: Consultancy; Janssen: Consultancy. Mato:LOXO: Honoraria, Research Funding; Johnson & Johnson: Honoraria, Research Funding; Genmab: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; DTRM Biopharma: Honoraria, Research Funding; Adaptive Biotechnologies: Honoraria; AbbVie: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Nurix: Research Funding; Pharmacyclics, LLC: Honoraria, Research Funding; TG Therapeutics, Inc: Honoraria, Research Funding; BeiGene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Curio: Honoraria; Pfizer: Research Funding; Octopharma: Honoraria, Research Funding; Dava: Honoraria; BMS: Honoraria; Medscape: Honoraria; Acerta: Research Funding; PER: Honoraria; PerView: Honoraria. Stilgenbauer:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Infinity: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Hoffmann-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sunesis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZenica: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Fehn:AstraZeneca: Current Employment, Current equity holder in private company. de Miranda:AstraZeneca: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Higgins:AstraZeneca: Current Employment; Palantir Technologies: Current equity holder in publicly-traded company; Rocket Companies: Current equity holder in publicly-traded company. John:AstraZeneca: Current Employment; University of Leicester: Ended employment in the past 24 months. de Borja:AstraZeneca: Current Employment, Current equity holder in publicly-traded company; Roche: Current equity holder in publicly-traded company. Jurczak:Celgene: Research Funding; TG Therapeutics: Research Funding; Bayer: Research Funding; Beigene: Consultancy, Research Funding; Sandoz: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Mei Pharma: Research Funding; Merck: Research Funding; Lilly: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Takeda: Research Funding; Loxo Oncology: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Morphosys: Research Funding; Novo Nordisk: Research Funding. Woyach:Newave: Consultancy; Janssen: Consultancy; Genentech: Consultancy; Pharmacyclics: Consultancy; MorphoSys: Consultancy, Research Funding; Schrodinger: Research Funding; Loxo@Lilly: Research Funding; BeiGene: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy, Research Funding; ArQule: Consultancy; Karyopharm Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal